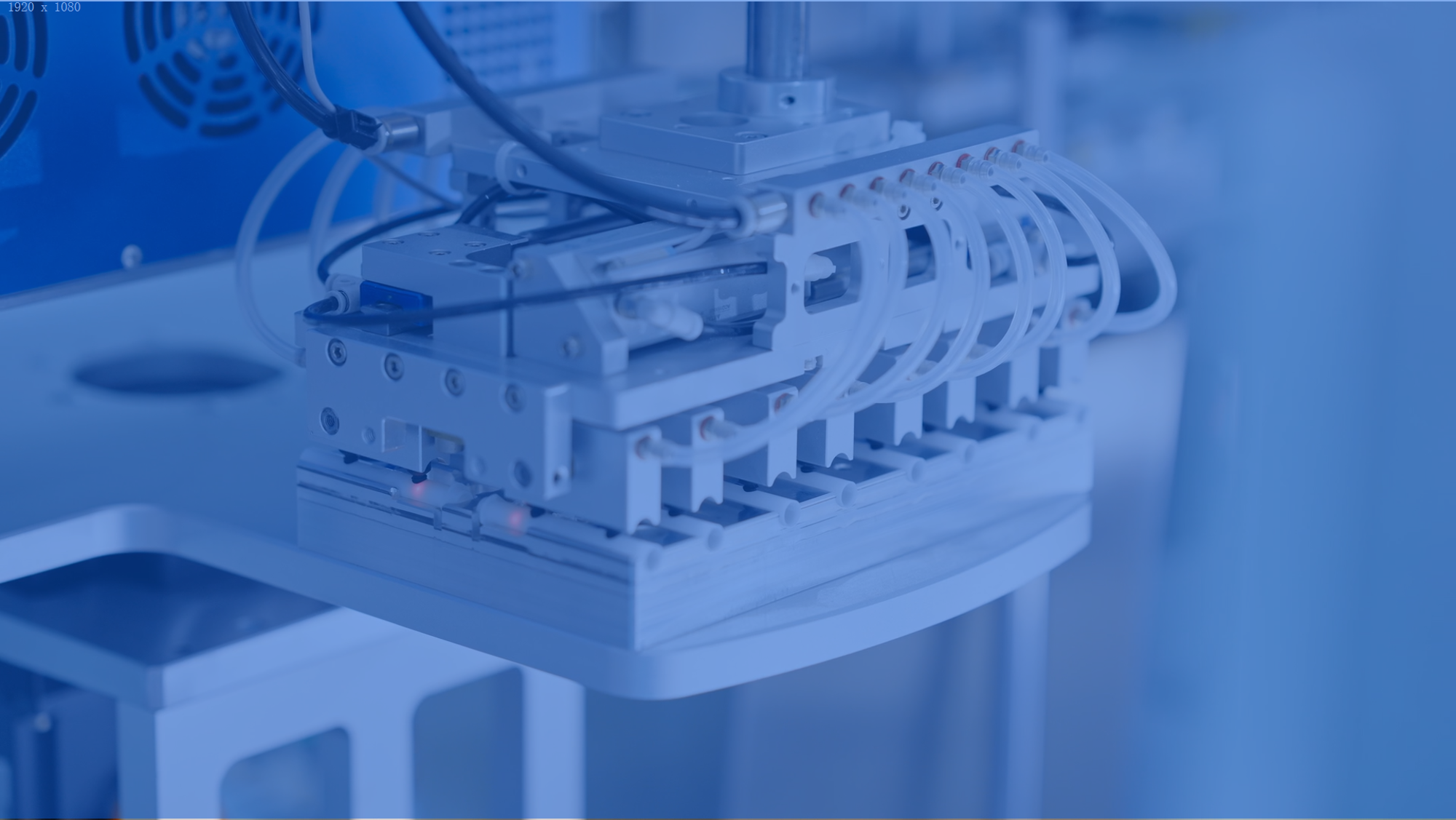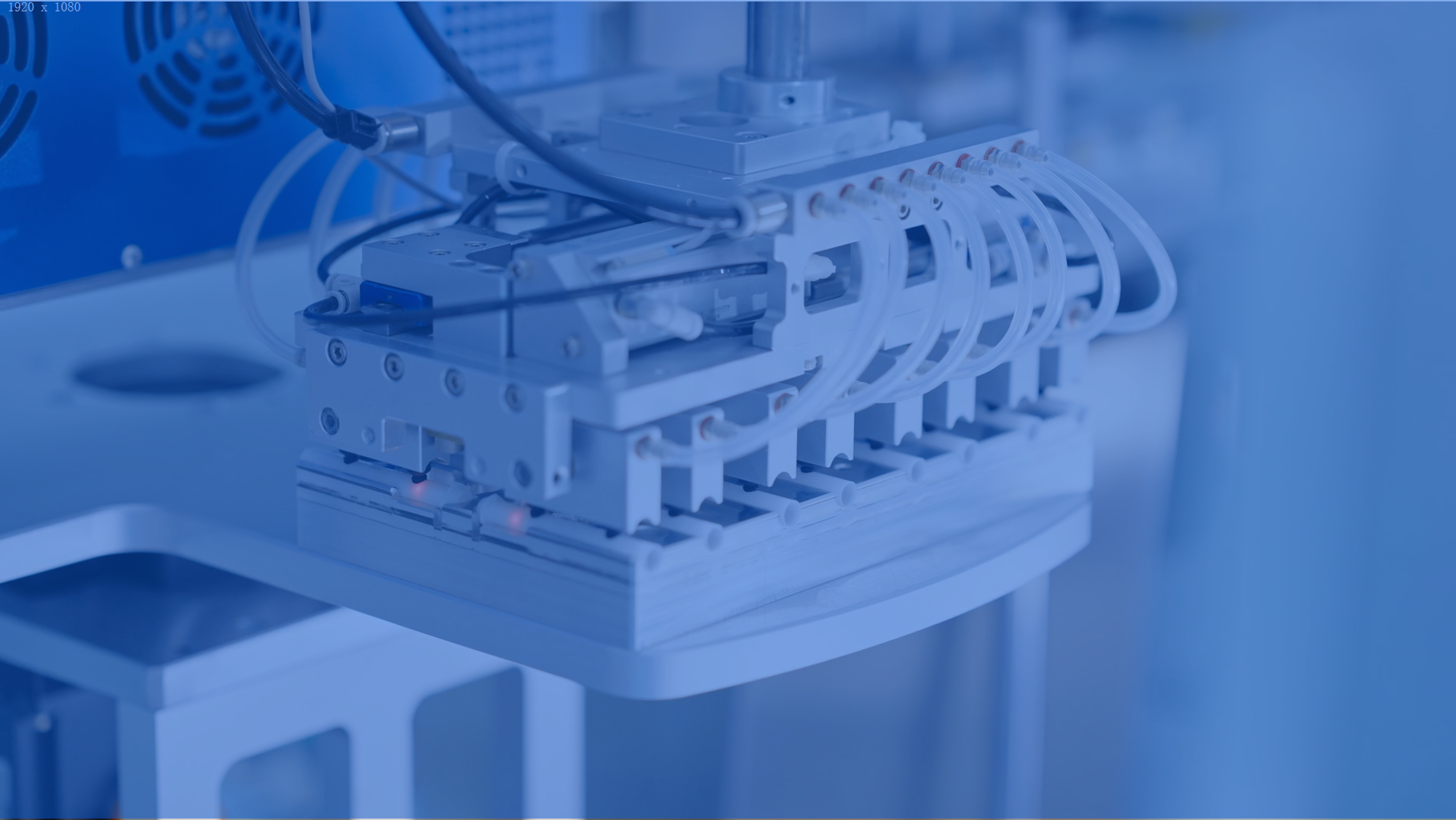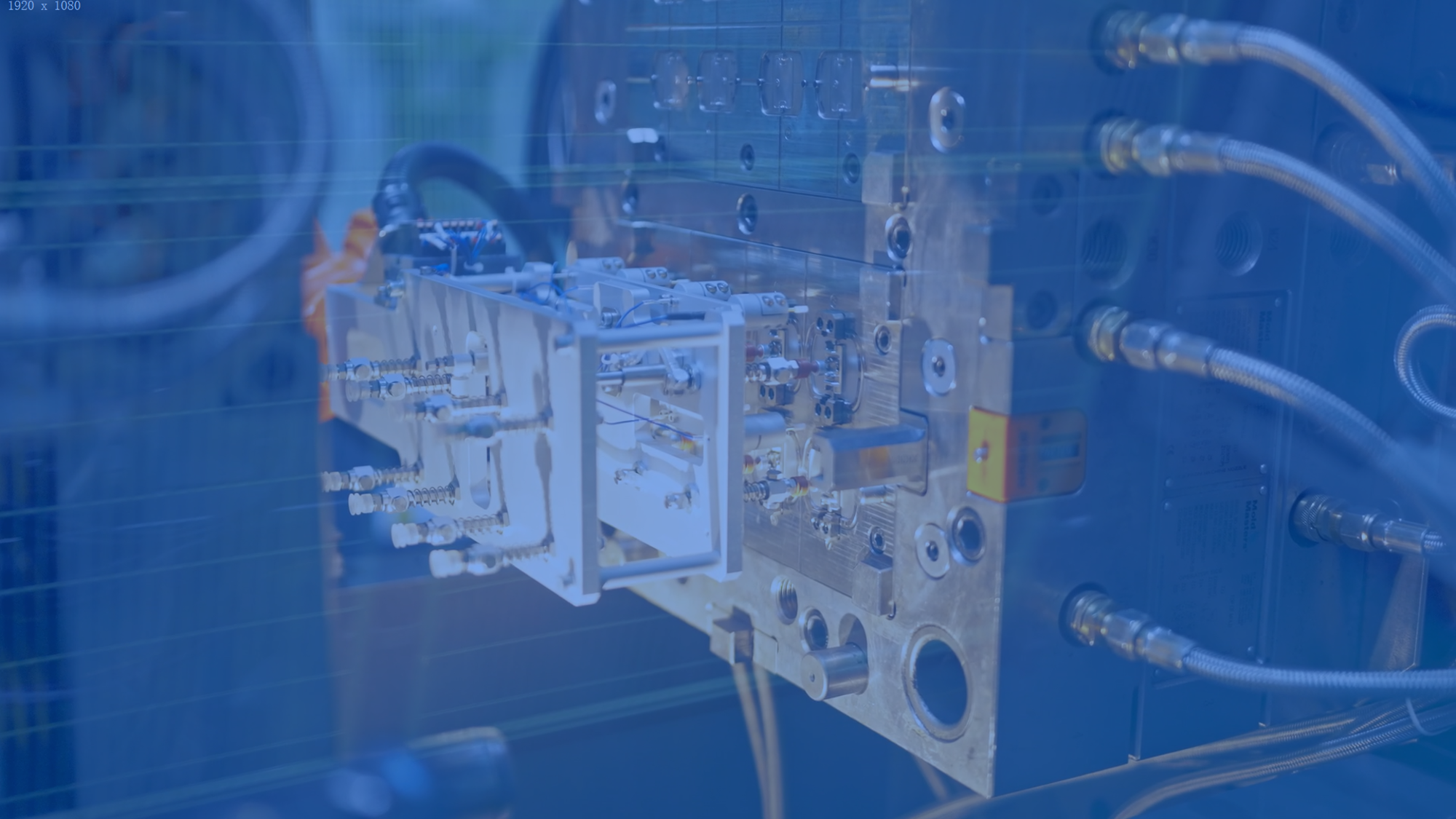
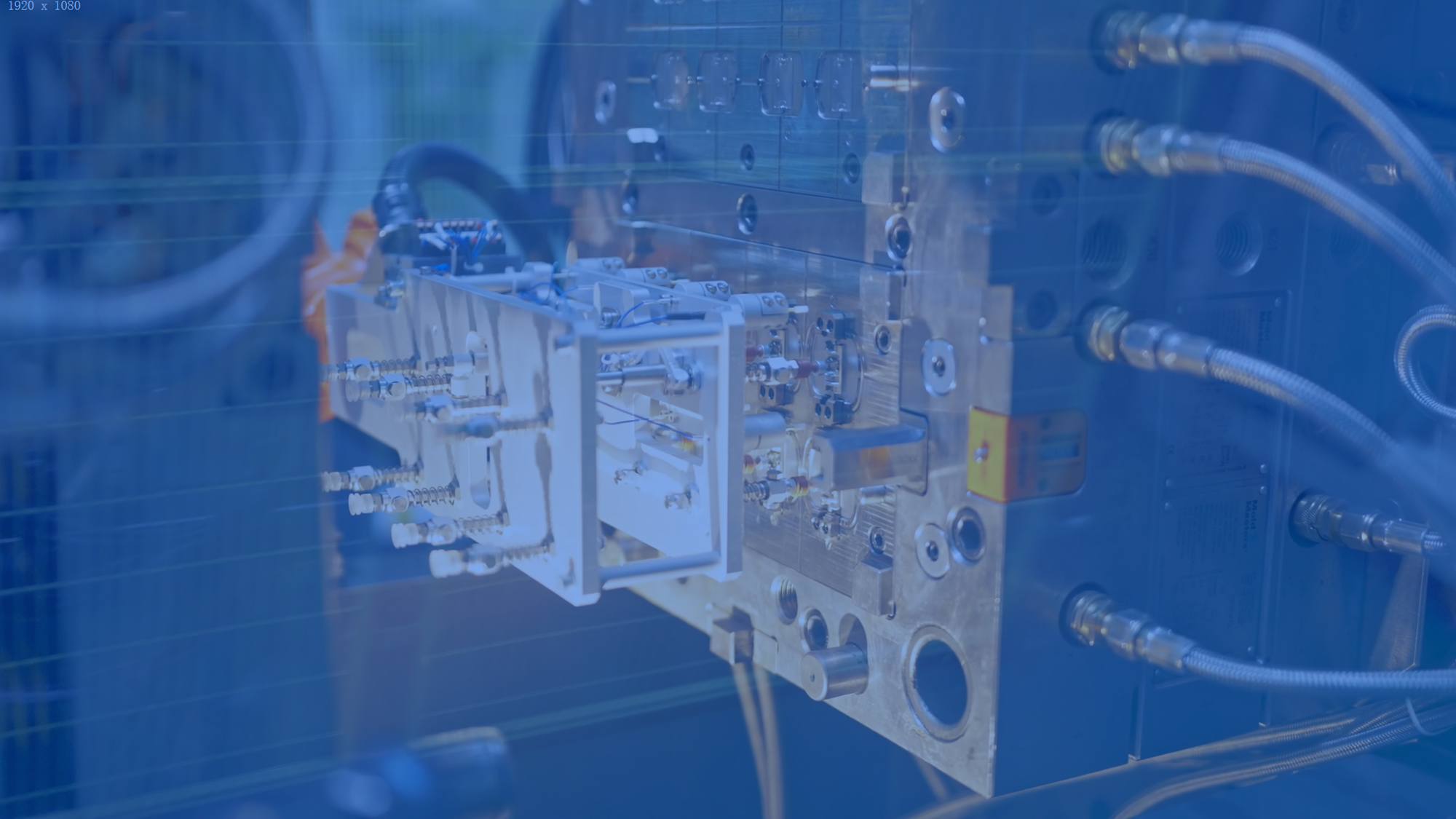
Tooling development
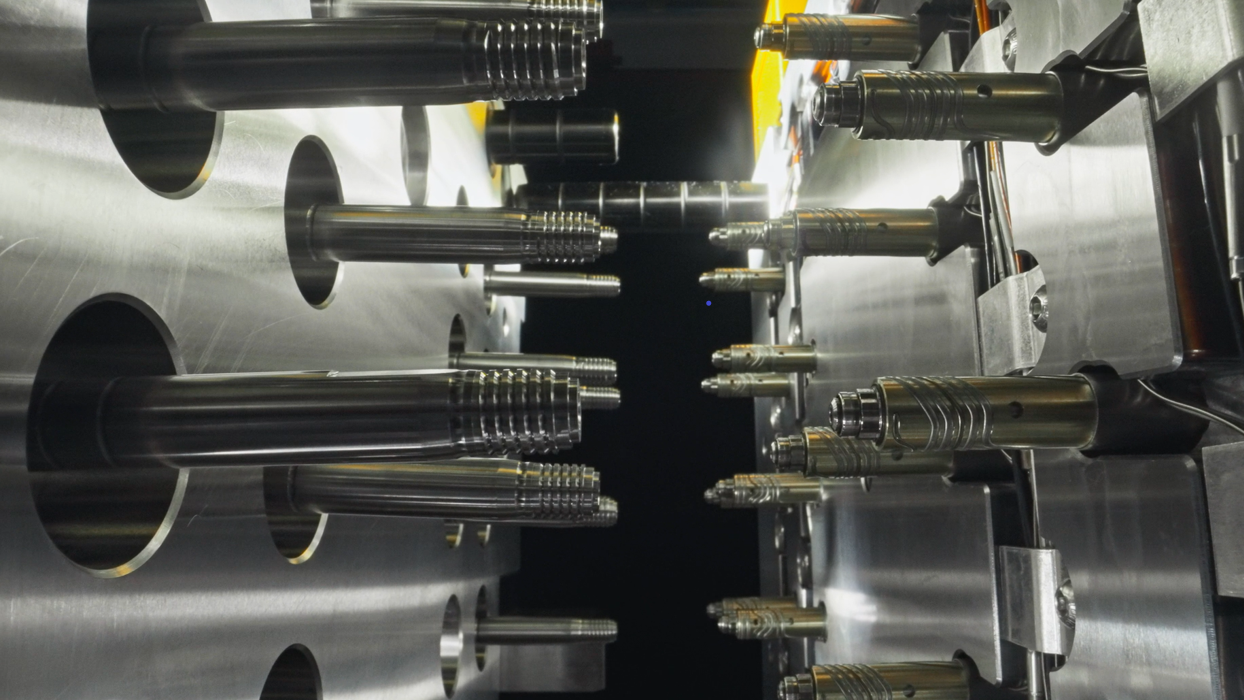
MeHow Medical possesses comprehensive mold manufacturing capabilities, utilizing advanced technologies such as high-precision CNC machining, EDM, and wire-cutting processes, achieving machining tolerances up to ±0.02mm.
With mature mold development and production experience, we provide reliable support for subsequent mass production scalability.
With mature mold development and production experience, we provide reliable support for subsequent mass production scalability.
Injection Molding
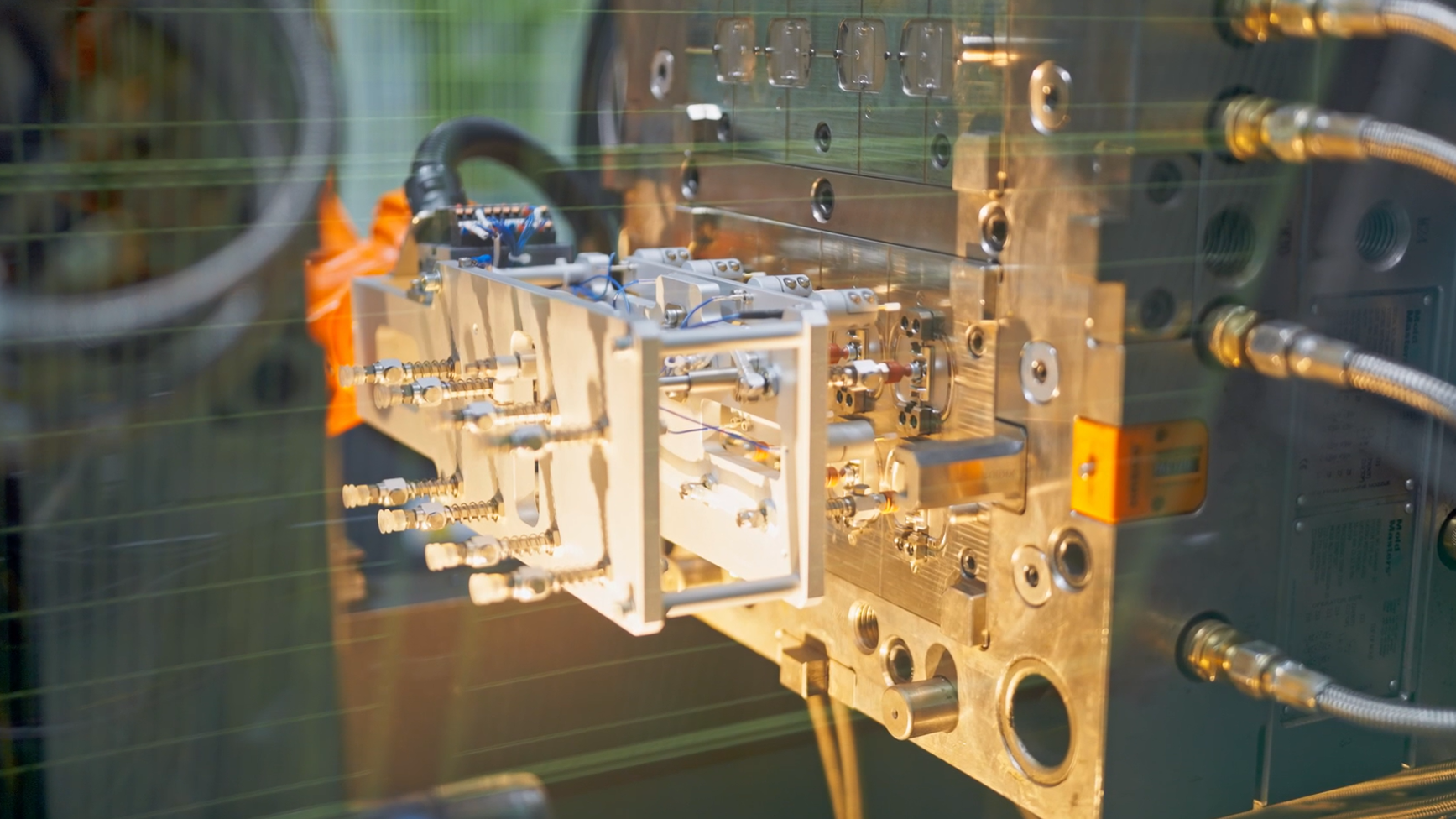
We master multi-cavity molds, insert molding, and two-shot molding technologies. Integrated automation systems enable rapid-cycle production of plastic and LSR components, driving significant efficiency gains and cost reduction.
ISBM
Utilizing industry-leading single-step ISBM technology, we complete injection, stretching, and blowing processes within a single workstation. This approach not only boosts productivity and shortens cycles but ensures exceptional dimensional accuracy with uniform wall thickness distribution.
Extrusion & Tubing
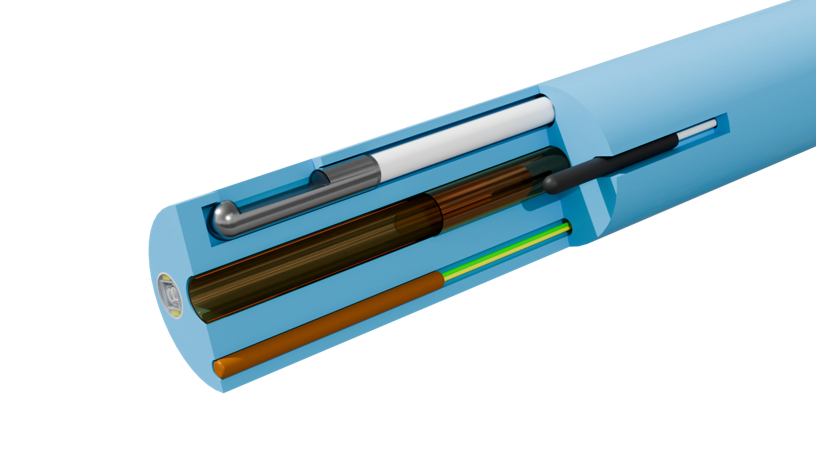
Medical catheter performance breakthroughs start with precision extrusion. Leveraging micron-level manufacturing technologies, we deliver complete solutions from minimally invasive devices to smart catheters – redefining performance standards at 0.01mm precision.
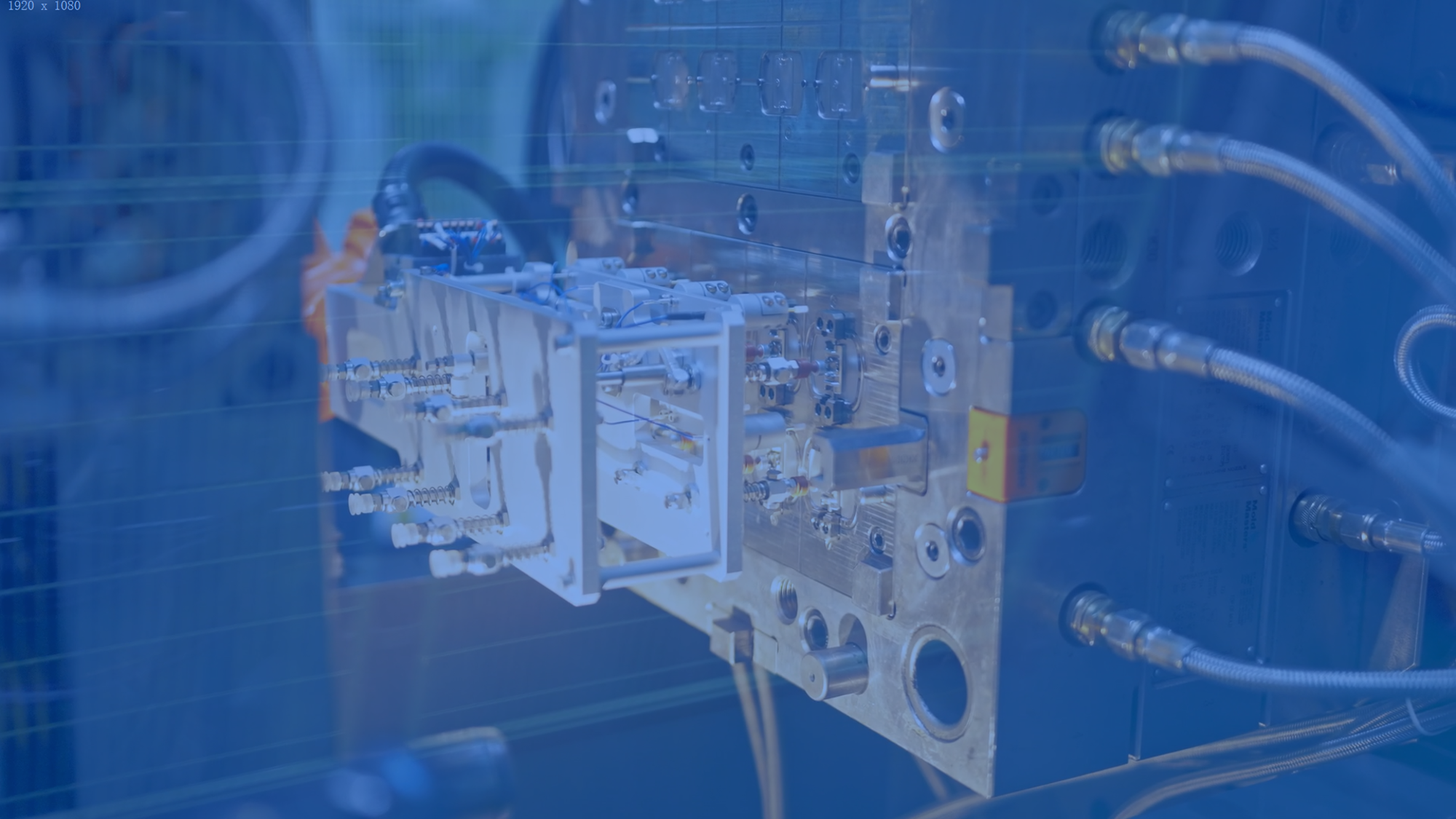
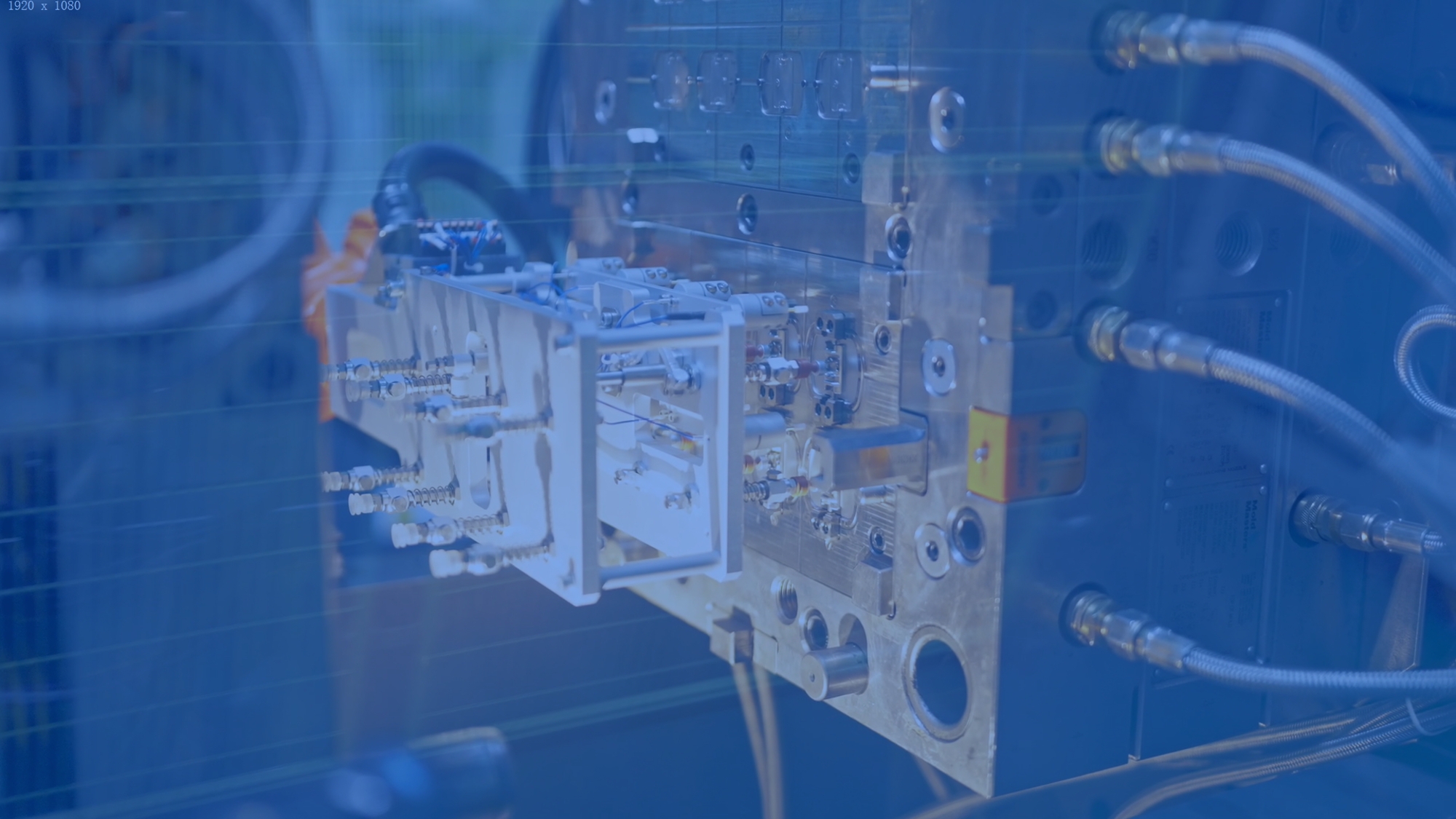
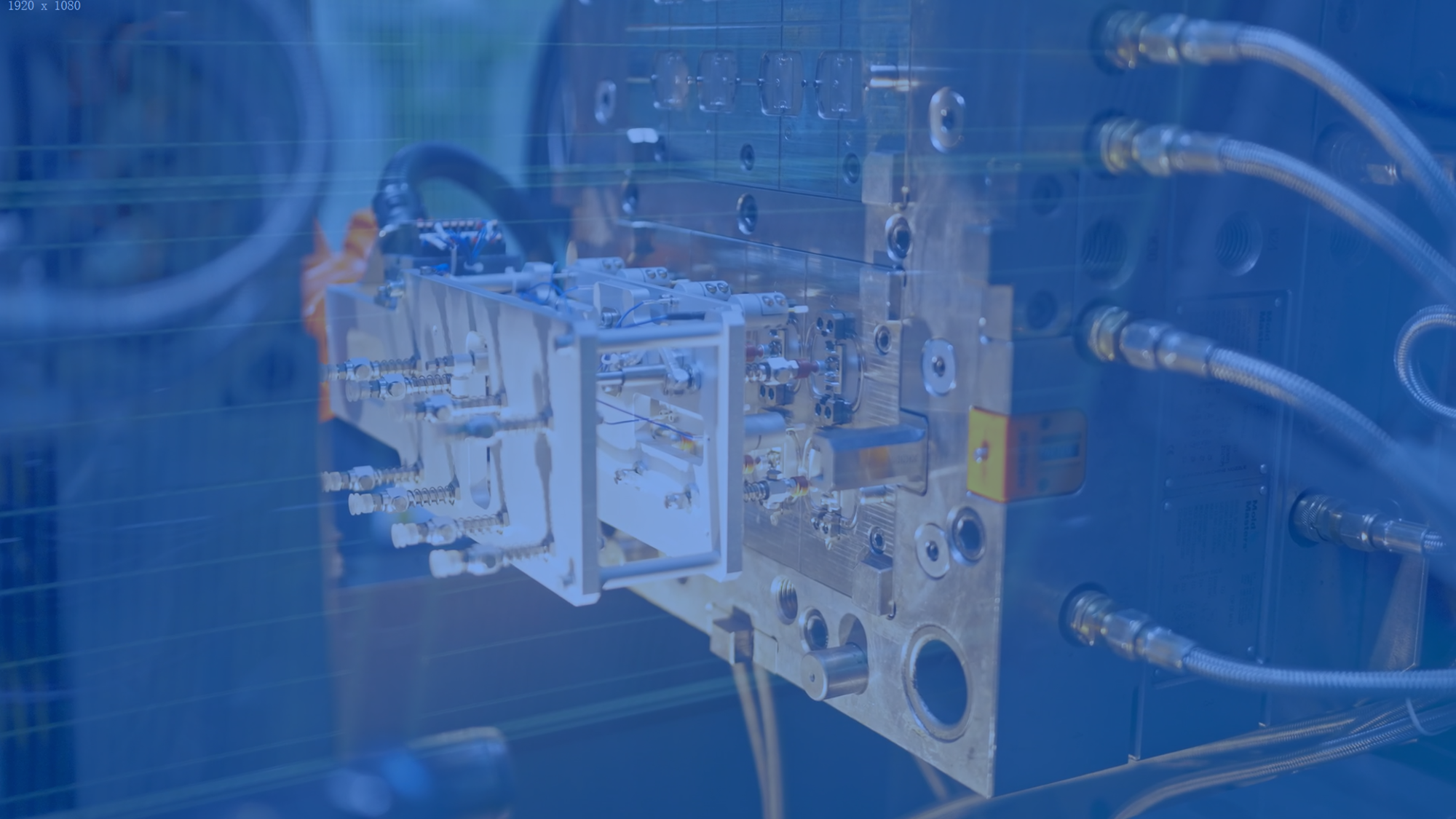
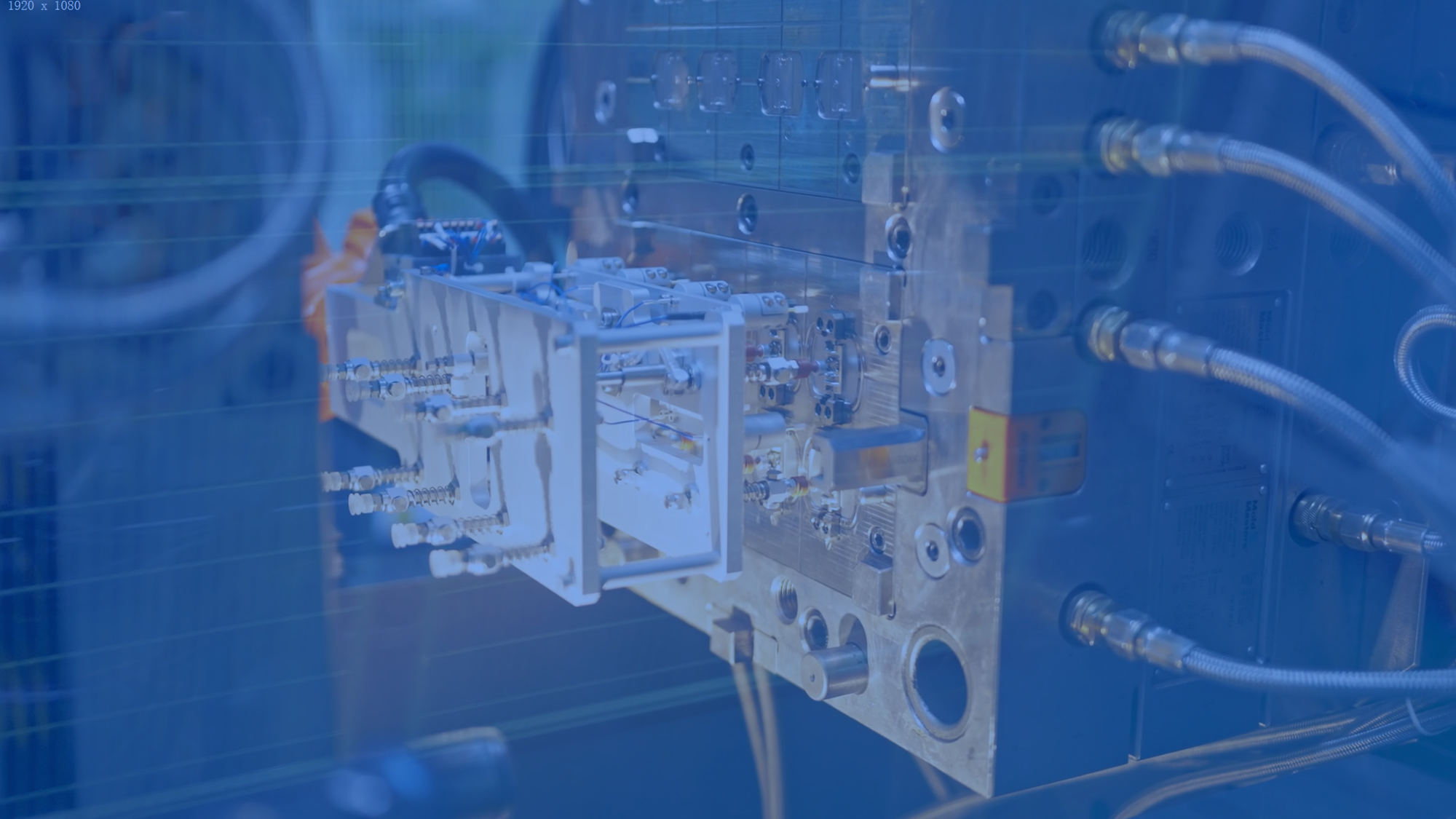
MEMs Technology
Successful sensor integration demands cross-disciplinary expertise and conquering engineering challenges in sensor development and platform packaging.
Our proven methodologies and industry insights pave the way for final-stage implementation.
Micro-Assembly
Medical-grade micro-assembly is critical for manufacturing high-end devices like cardiovascular interventions and implantable sensors. Our established micron-level assembly systems ensure clinical-grade precision and reliability.
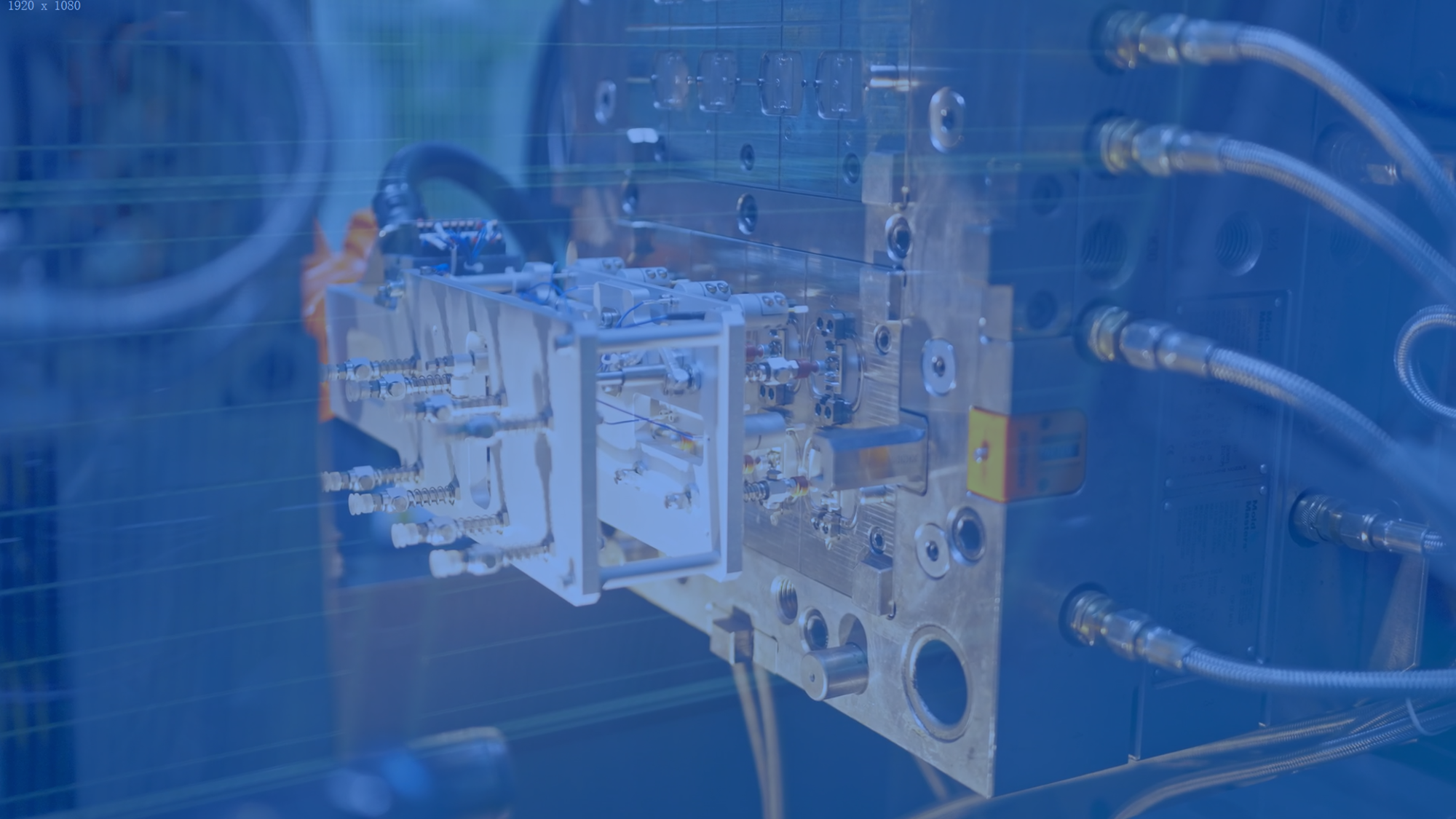
Assembly and Secondary Ops
Our secondary processes include ultrasonic welding, laser marking, plastic heat staking, and automated dispensing. These mature processes ensure optimal functional and cosmetic quality for finished devices.
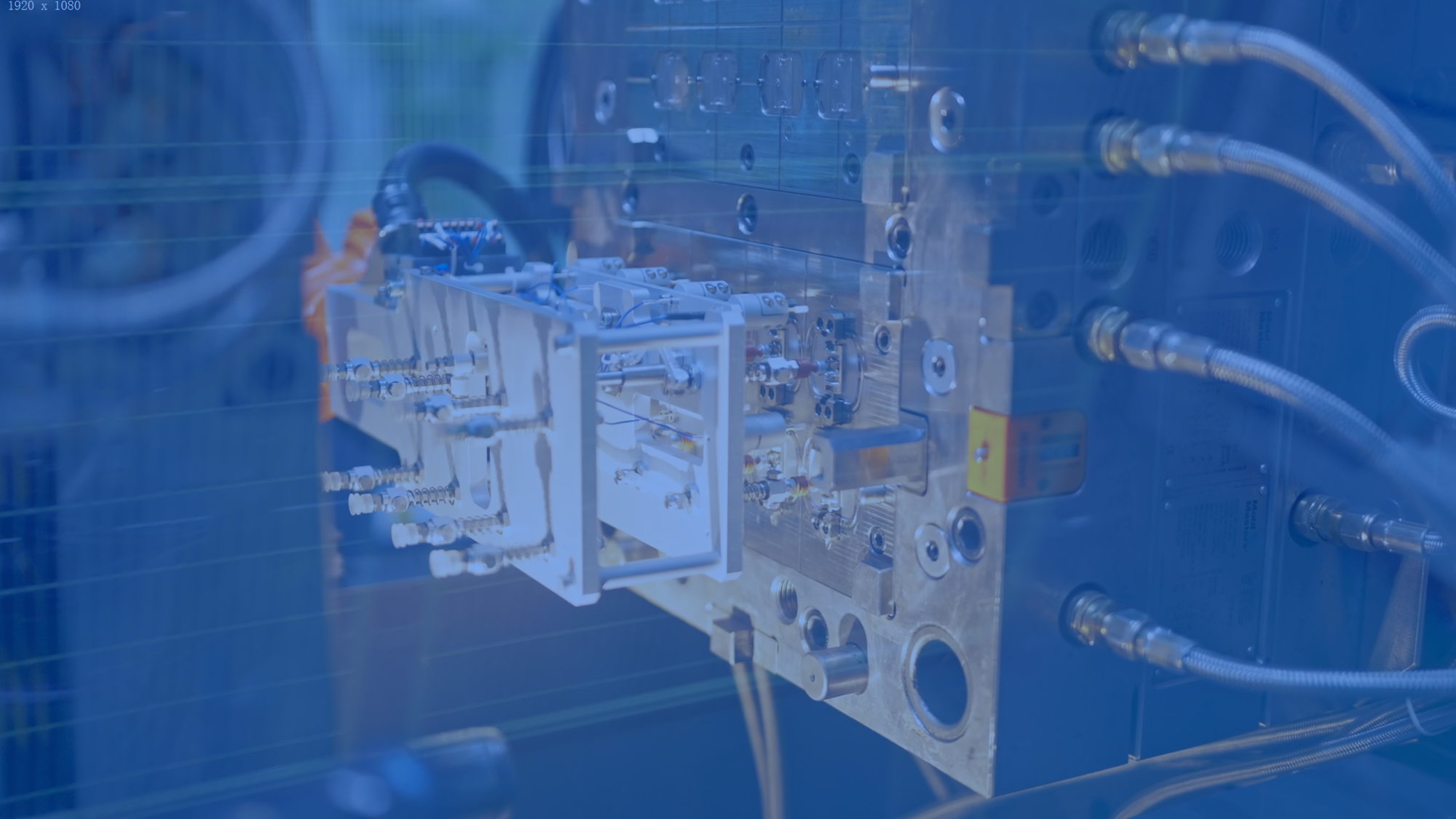
Packaging and Sterilization
We provide medical-grade packaging solutions featuring automated sealing and EO/radiation sterilization. Full compliance with ISO 11135 ensures sterile products meet global safety requirements.
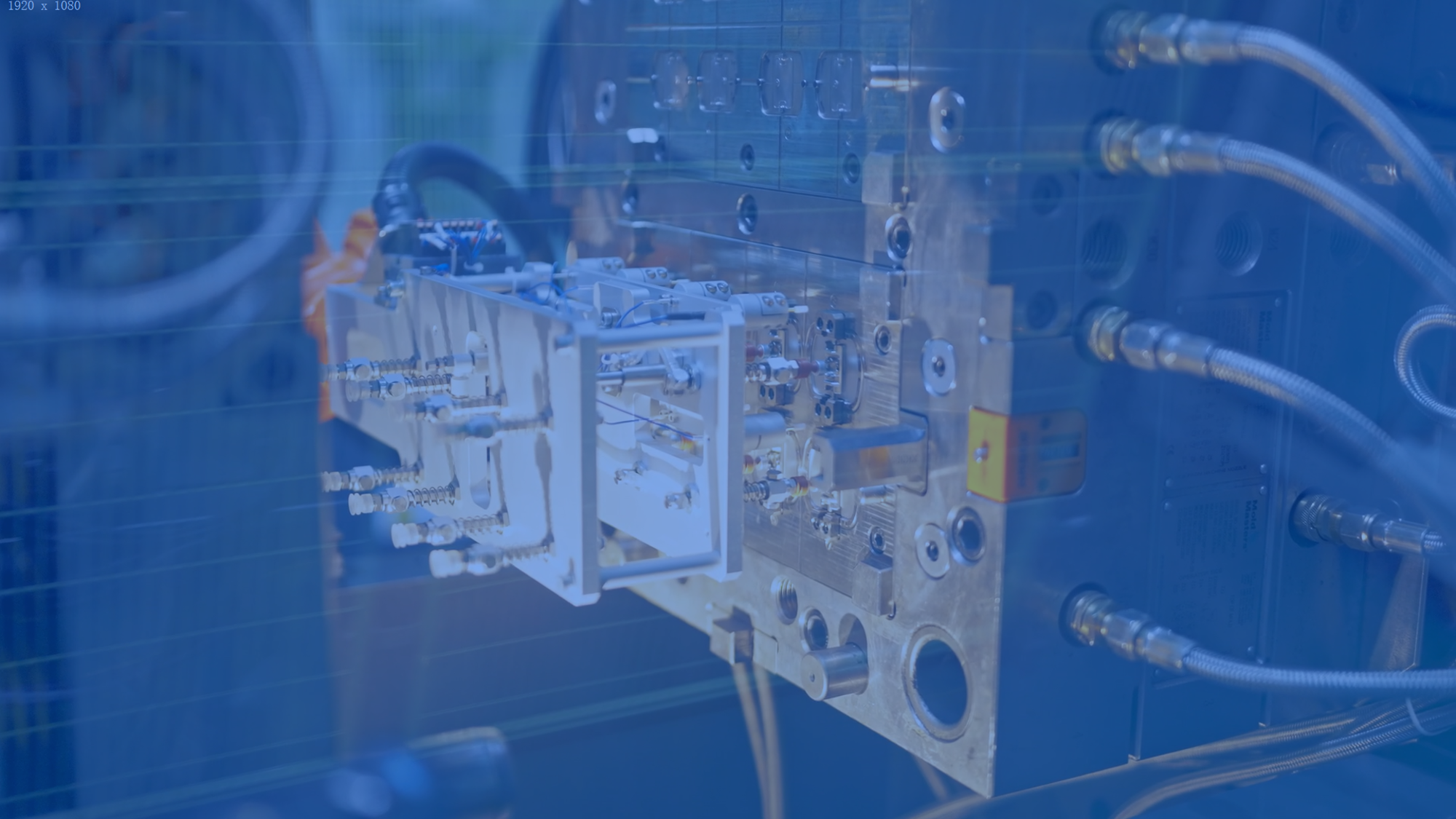
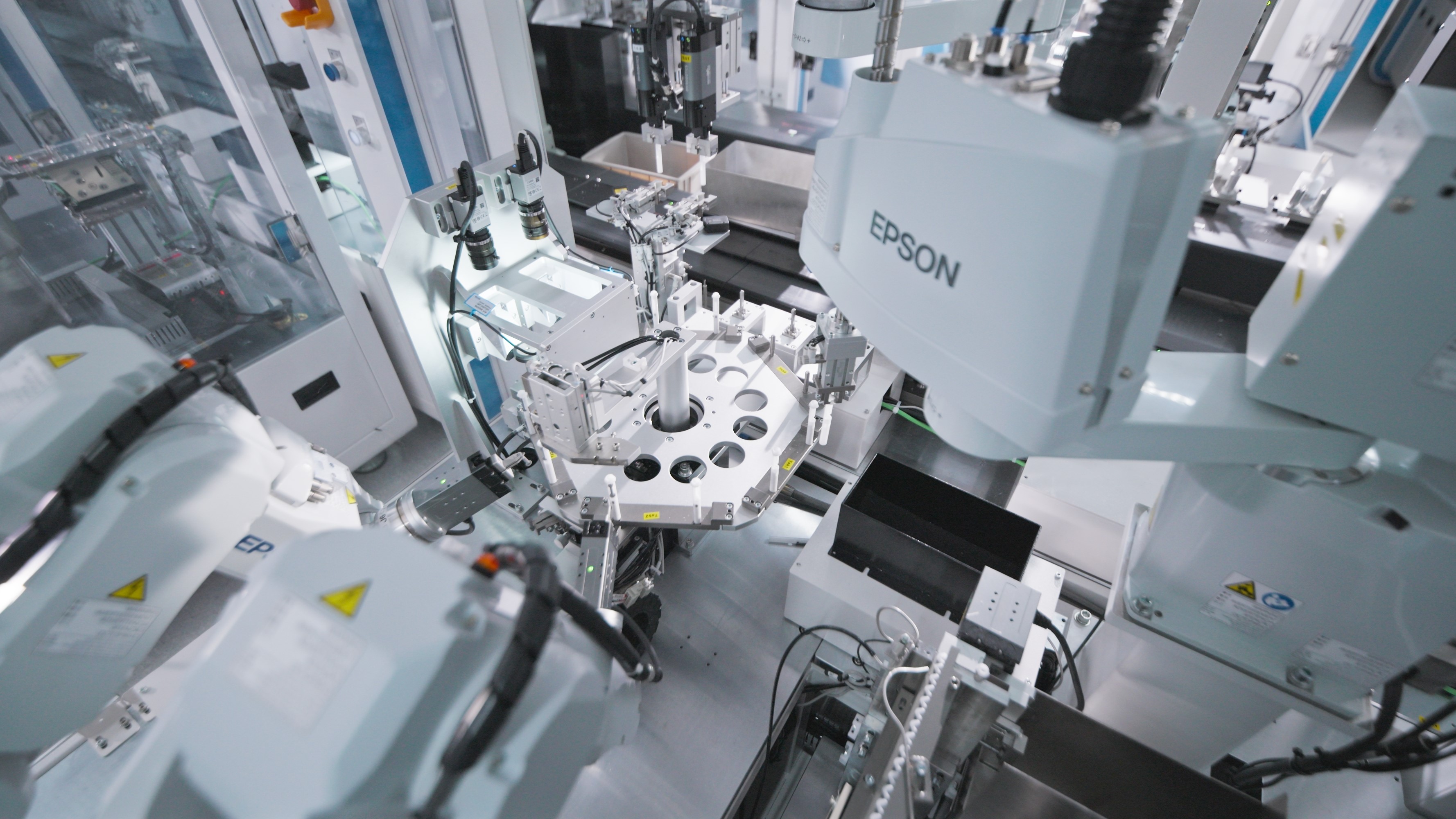
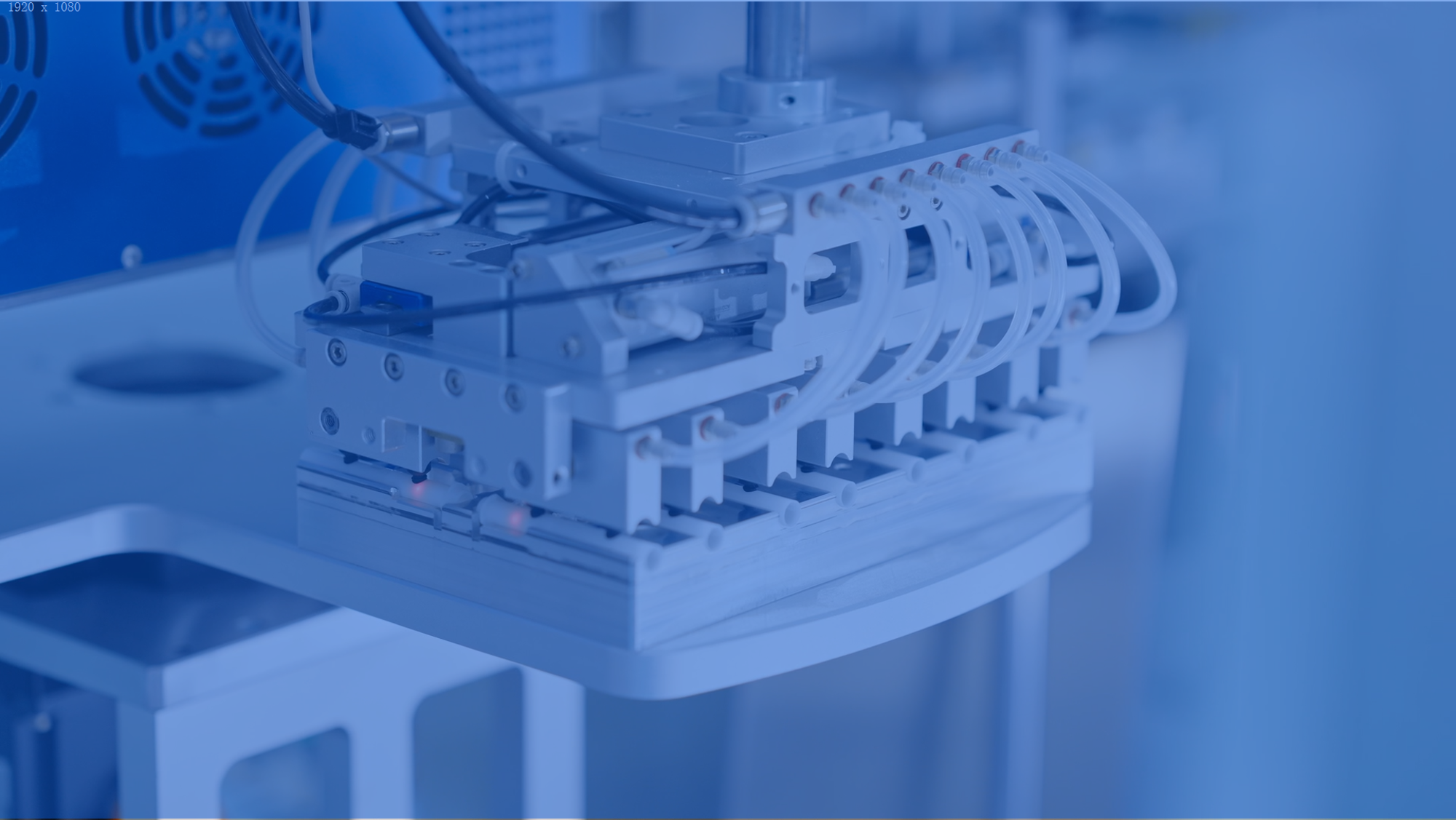
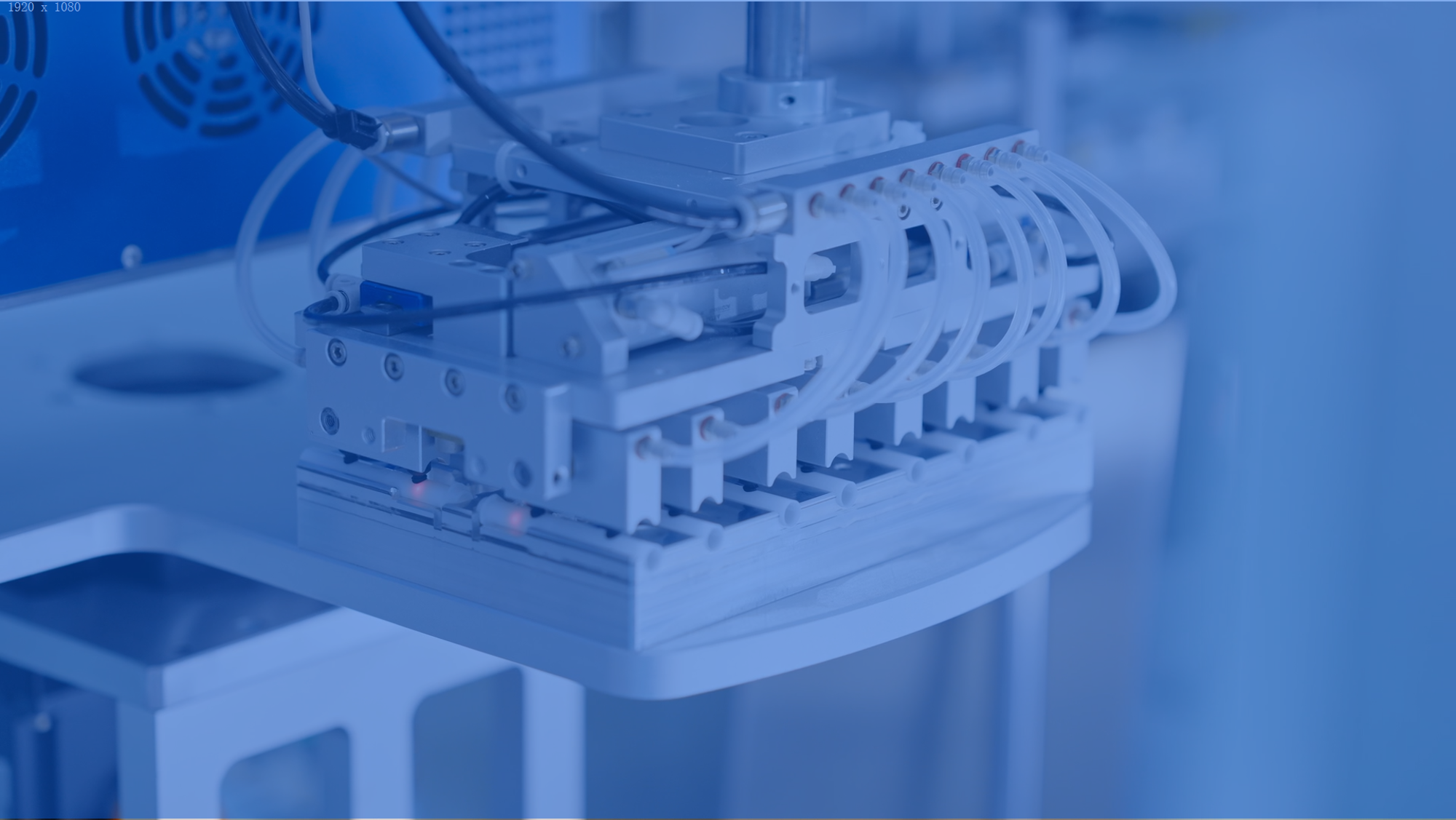
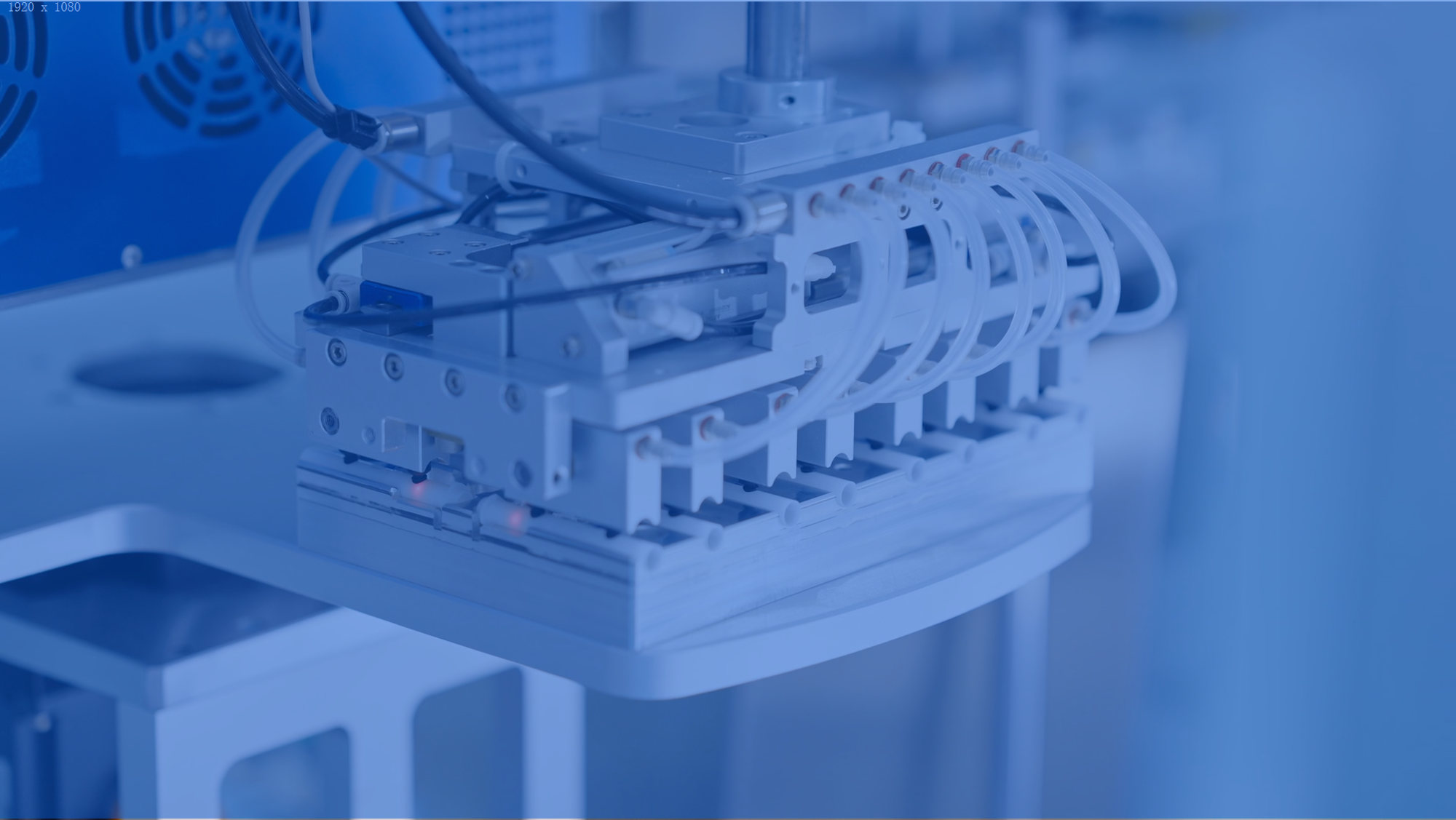
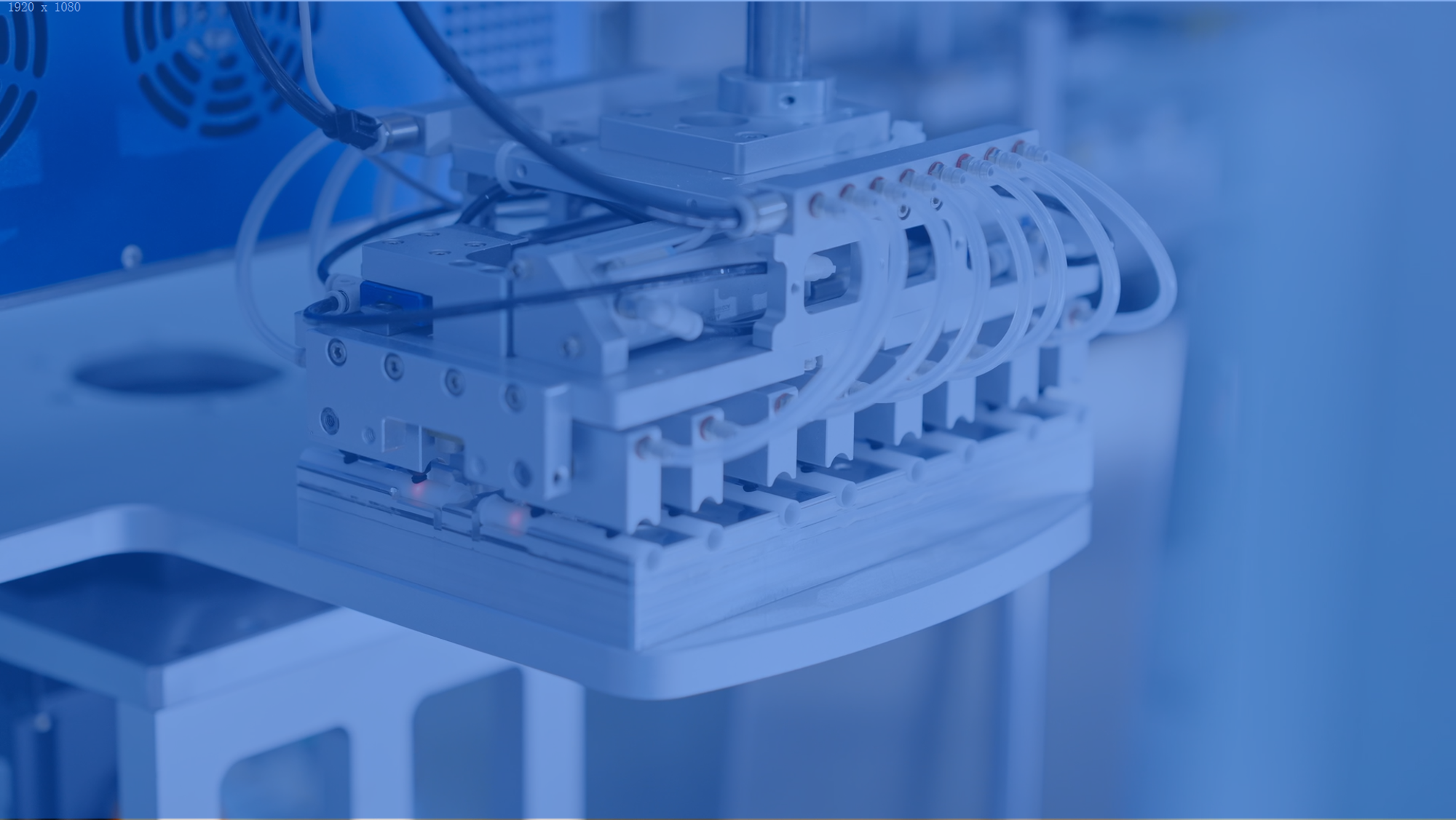
Automation
Mehow's intelligent production lines integrate AI-based vision systems, precision motion control platforms, and modular automation libraries. This ecosystem enables high-yield, scalable manufacturing for medical devices.