-
Company
Company
 CompanyWe uphold our mission of "continuous innovation for life and health," providing global healthcare clients with end-to-end solutions—from concept design to manufacturing—to boost product value.ESG
CompanyWe uphold our mission of "continuous innovation for life and health," providing global healthcare clients with end-to-end solutions—from concept design to manufacturing—to boost product value.ESG ESGMeHow deeply integrates ESG goals into strategic decision-making and daily operations. Explore our sustainable development management practices.
ESGMeHow deeply integrates ESG goals into strategic decision-making and daily operations. Explore our sustainable development management practices. -
Services
Design & Development
 Design & DevelopmentTransforming concepts into advanced, compliant, and high-value solutions through proprietary technologies and platforms.NPI
Design & DevelopmentTransforming concepts into advanced, compliant, and high-value solutions through proprietary technologies and platforms.NPI NPIA professional NPI system accelerates product industrialization, ensuring 100% conversion of design inputs into reliable delivery.Quality Management
NPIA professional NPI system accelerates product industrialization, ensuring 100% conversion of design inputs into reliable delivery.Quality Management Quality ManagementWe have developed a comprehensive quality control system that covers every step of the process, and stringent quality standards are embedded in each stage from raw materials to final product delivery.Program Management
Quality ManagementWe have developed a comprehensive quality control system that covers every step of the process, and stringent quality standards are embedded in each stage from raw materials to final product delivery.Program Management Program ManagementAn experienced management team and agile project management ensure the uncompromised achievement of your project's triple objectives: compliance, cost, and schedule.Testing
Program ManagementAn experienced management team and agile project management ensure the uncompromised achievement of your project's triple objectives: compliance, cost, and schedule.Testing Inspection & Testing CapabilityMultiple testing capabilities support product and process validation, including surface properties testing, reliability testing, biological testing, and Bisphenol A (BPA) testing.Clinical and Registration
Inspection & Testing CapabilityMultiple testing capabilities support product and process validation, including surface properties testing, reliability testing, biological testing, and Bisphenol A (BPA) testing.Clinical and Registration Clinical and RegistrationWe provide regulatory registration and submission services, assist with clinical trial support, and handle technical documentation to secure CE and FDA approvals.Supply Chain
Clinical and RegistrationWe provide regulatory registration and submission services, assist with clinical trial support, and handle technical documentation to secure CE and FDA approvals.Supply Chain Supply ChainYour end-to-end solution for the medical device lifecycle—optimizing supply chain complexity.MAH
Supply ChainYour end-to-end solution for the medical device lifecycle—optimizing supply chain complexity.MAH MAH ServicesWith a 400,000 m² smart manufacturing campus and an experienced regulatory team, we empower clients to operate with a light-asset model, scale rapidly, and meet global compliance standards.
MAH ServicesWith a 400,000 m² smart manufacturing campus and an experienced regulatory team, we empower clients to operate with a light-asset model, scale rapidly, and meet global compliance standards. -
Capabilities
Tooling Development
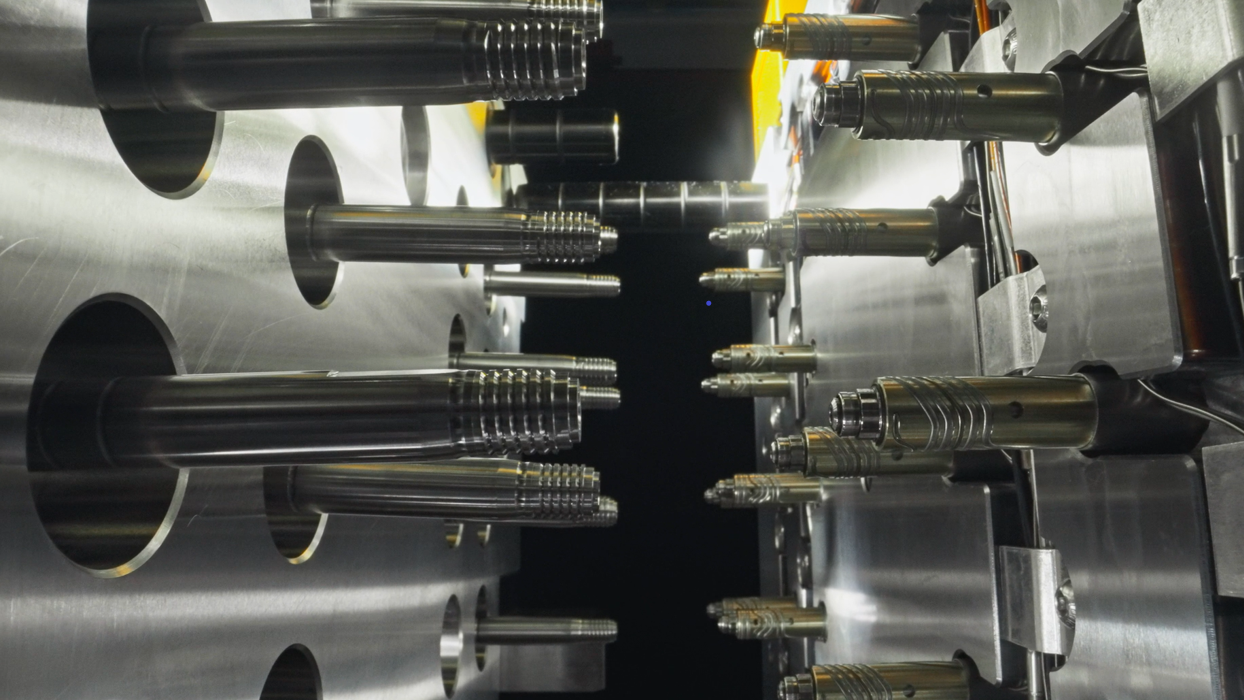 Mold ManufacturingMehow focusing on micron-level precision to drive improvements in both production efficiency and product quality.Injection Molding
Mold ManufacturingMehow focusing on micron-level precision to drive improvements in both production efficiency and product quality.Injection Molding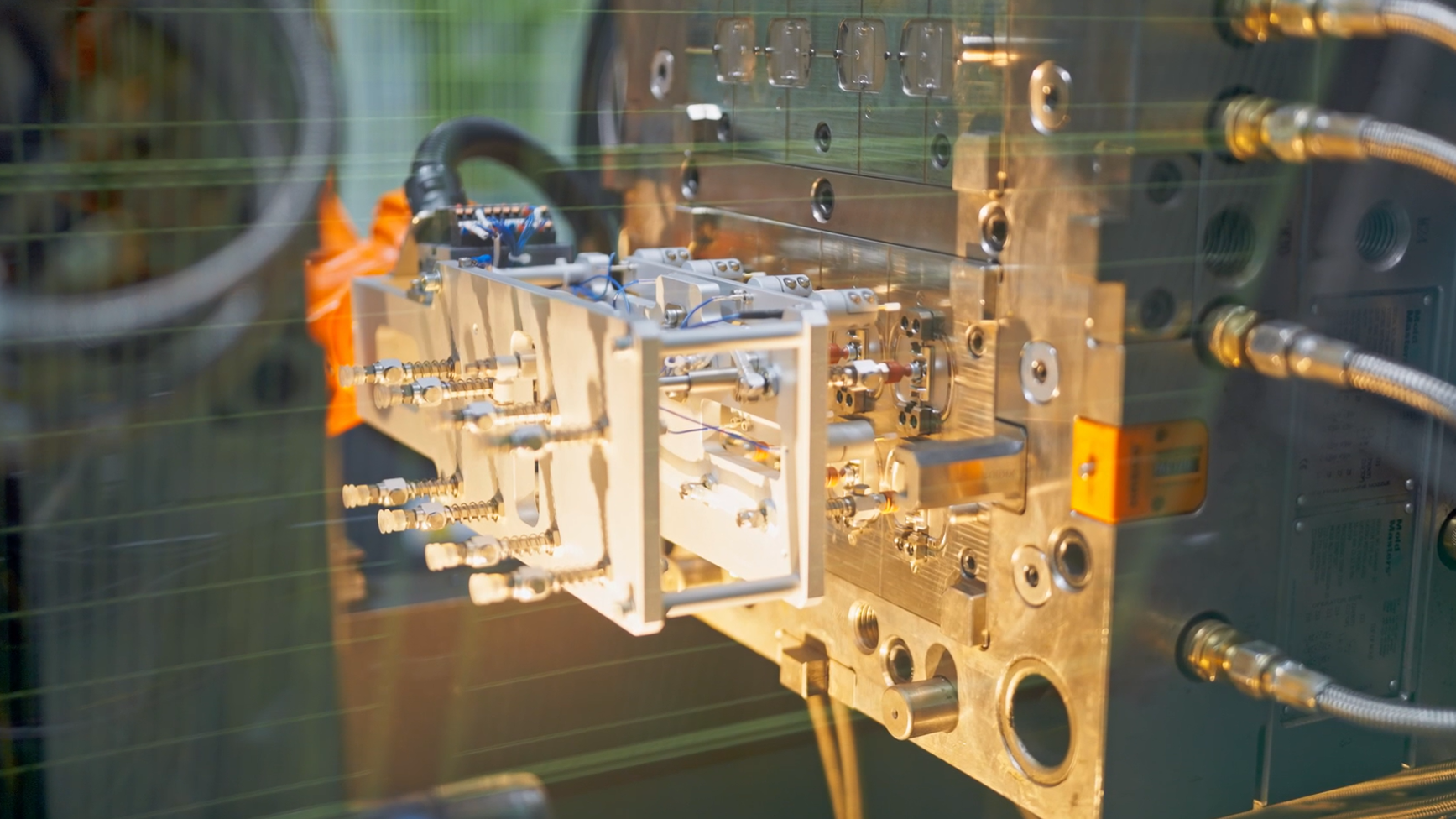 Injection MoldingWith mature materials and precision injection moulding technology, MEHOW offer plastic injection moulding, liquid silicone injection moulding, micro injection moulding and other moulding solutions.ISBM
Injection MoldingWith mature materials and precision injection moulding technology, MEHOW offer plastic injection moulding, liquid silicone injection moulding, micro injection moulding and other moulding solutions.ISBM ISBMRelying on one-step injection stretch blow moulding technology and high-quality PET materials, MEHOW focus on creating high-performance pharmaceutical packaging containers.Extrusion and Tubing
ISBMRelying on one-step injection stretch blow moulding technology and high-quality PET materials, MEHOW focus on creating high-performance pharmaceutical packaging containers.Extrusion and Tubing Extrusion and TubingLeveraging our extensive expertise in extrusion and tubing manufacturing, we deliver comprehensive extrusion solutions spanning from minimally invasive interventions to smart catheters, redefining the performance boundaries of catheters through micron-level precision control.MEMs Technology
Extrusion and TubingLeveraging our extensive expertise in extrusion and tubing manufacturing, we deliver comprehensive extrusion solutions spanning from minimally invasive interventions to smart catheters, redefining the performance boundaries of catheters through micron-level precision control.MEMs Technology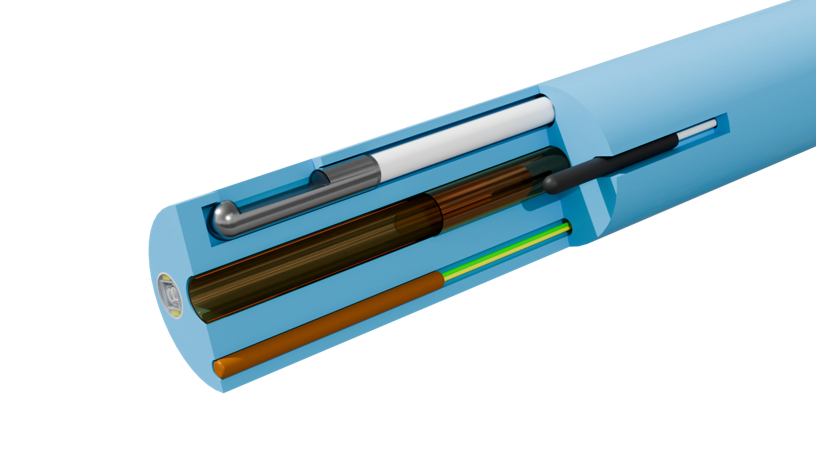 MEMs TechnologyProfessional Sensor Integration Services: Designing, customizing, and packaging temperature & magnetic sensors for high-density, high-reliability integration within the confined spaces of minimally invasive devices.Micro Assembly
MEMs TechnologyProfessional Sensor Integration Services: Designing, customizing, and packaging temperature & magnetic sensors for high-density, high-reliability integration within the confined spaces of minimally invasive devices.Micro Assembly Micro-Assembly SolutionsAnnular electrode welding, micro-assembly dispensing, and scalable processes enabling flexible production from low to high volumes.Assembly and Secondary Ops
Micro-Assembly SolutionsAnnular electrode welding, micro-assembly dispensing, and scalable processes enabling flexible production from low to high volumes.Assembly and Secondary Ops Post-Processing and AssemblyFunctional surface reinforcement, customised appearance processes and full-process value-added development services.Packaging and Sterilization
Post-Processing and AssemblyFunctional surface reinforcement, customised appearance processes and full-process value-added development services.Packaging and Sterilization Packaging & SterilizationComprehensive Packaging Services: Design & Development, Process Validation, Sterilization, and Testing.Automation
Packaging & SterilizationComprehensive Packaging Services: Design & Development, Process Validation, Sterilization, and Testing.Automation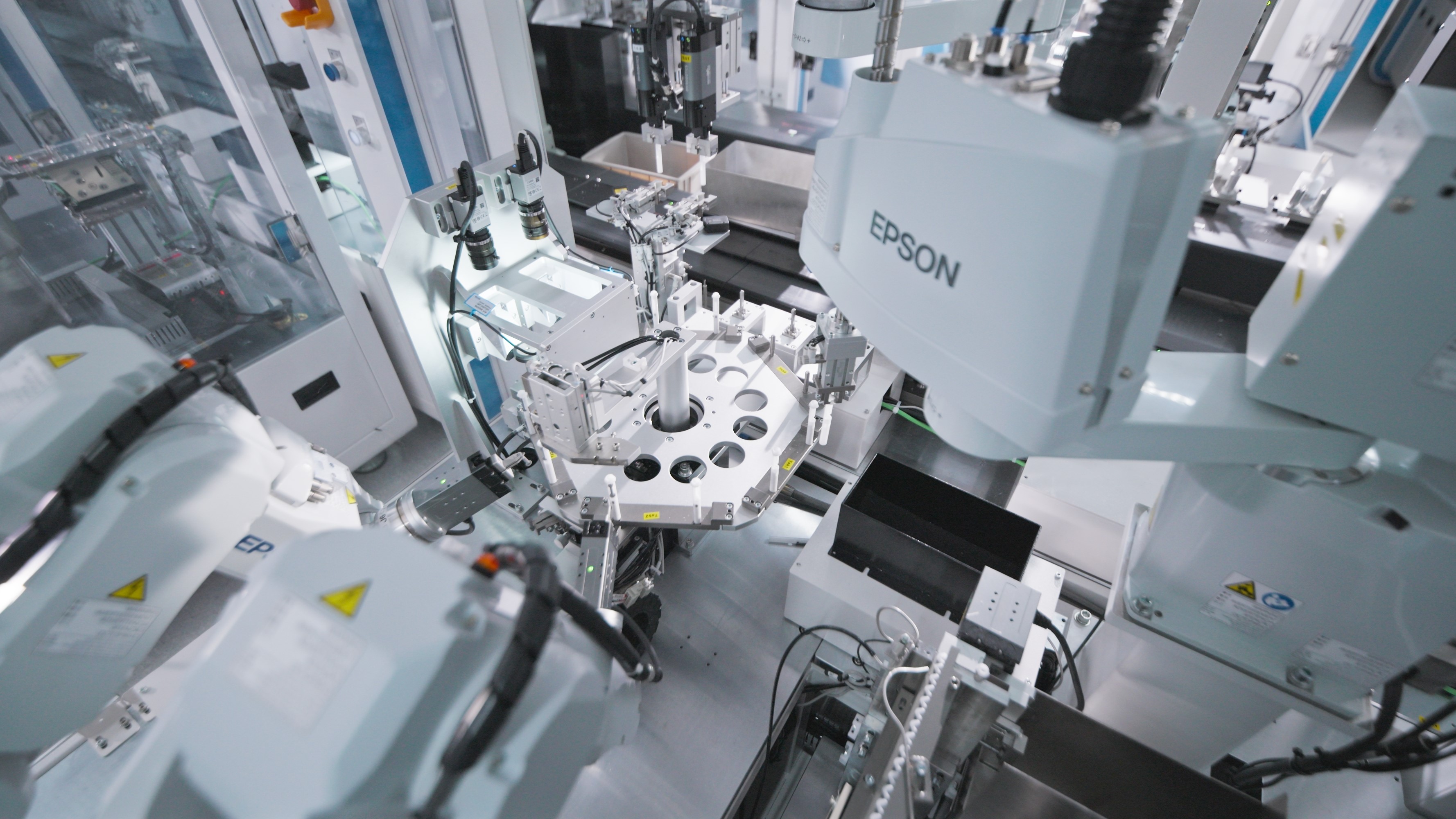 AutomationEnd-to-end automation solutions driving transformative productivity gains.
AutomationEnd-to-end automation solutions driving transformative productivity gains. -
Solutions
Drug Delivery
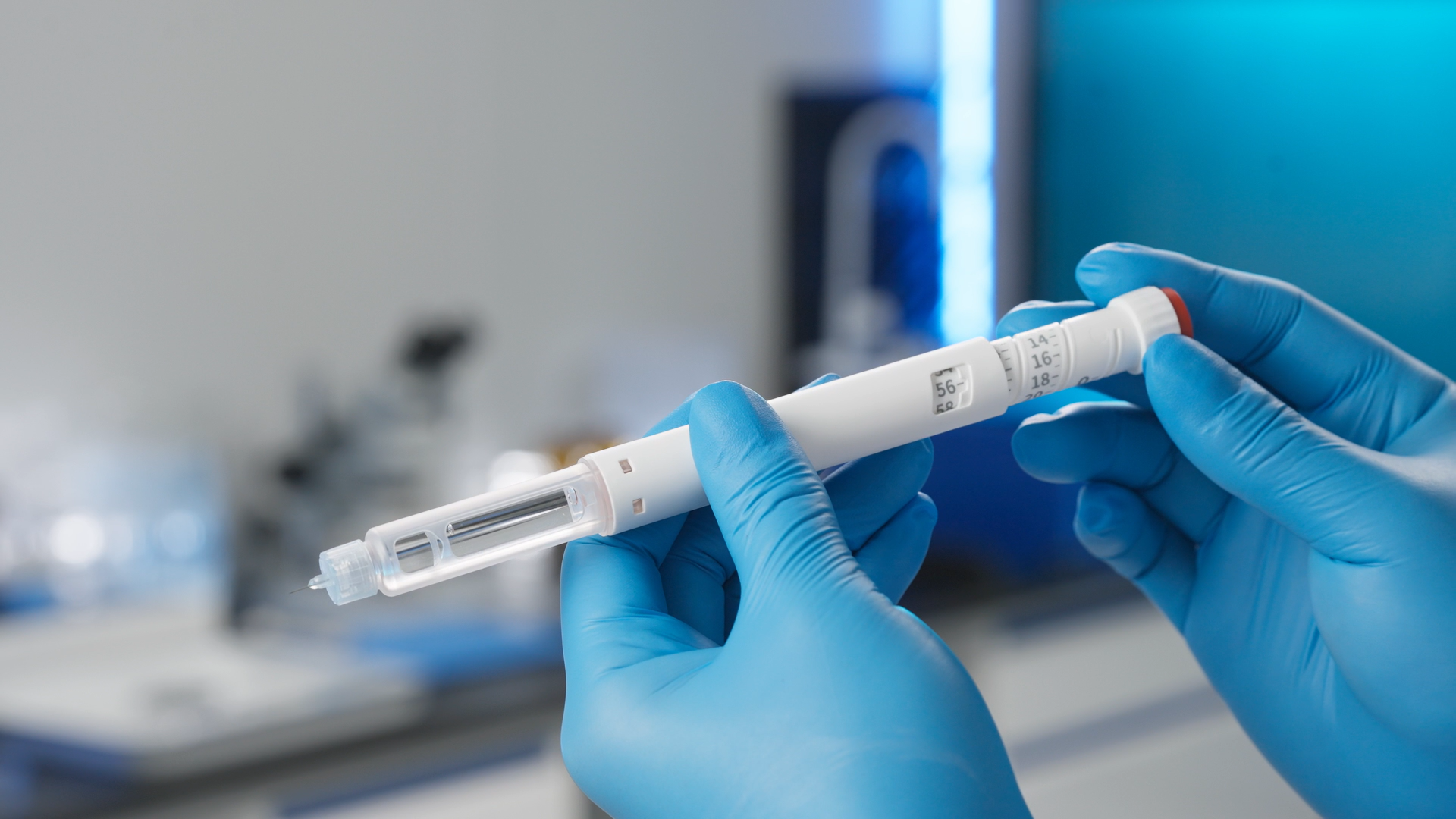 Drug DeliveryOne-stop drug delivery solutions including Variable Dose Pen, Auto Injector, and Auto Secure Dose Pen.In Vitro Diagnostics
Drug DeliveryOne-stop drug delivery solutions including Variable Dose Pen, Auto Injector, and Auto Secure Dose Pen.In Vitro Diagnostics In Vitro DiagnosticsMicrofluidics, Laboratory Consumables, Reagent Packaging.Cardiovascular
In Vitro DiagnosticsMicrofluidics, Laboratory Consumables, Reagent Packaging.Cardiovascular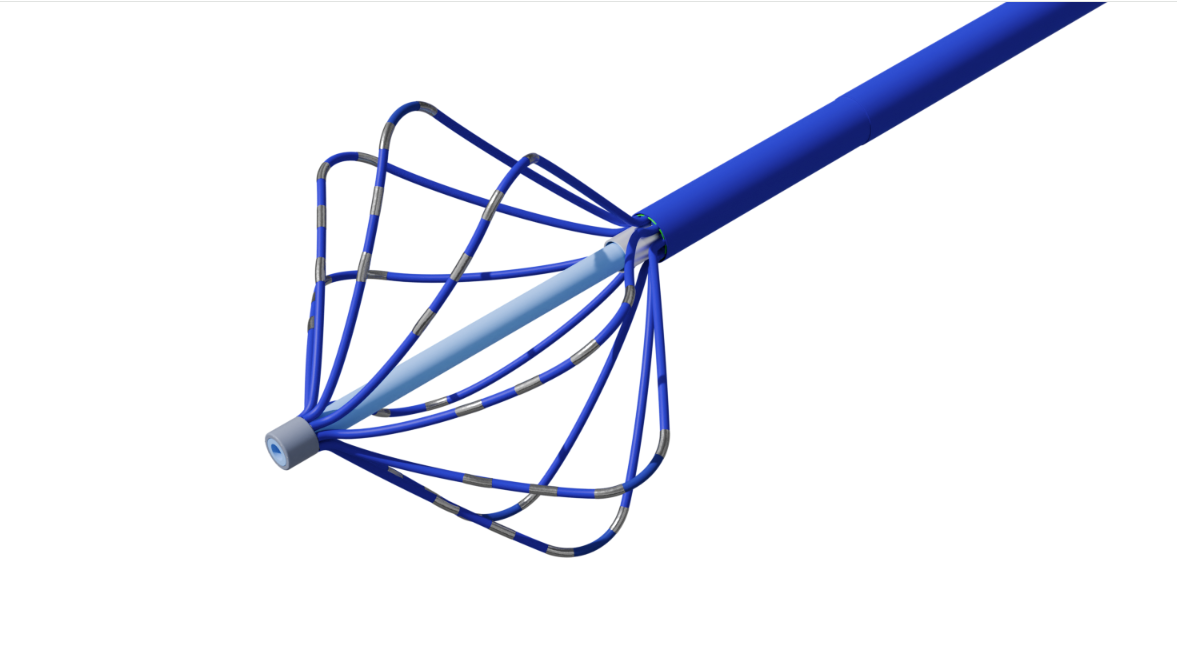 CardiovascularElectrophysiology (EP) Catheter Solutions: Ablation Catheters, High-Density Mapping Catheters, and Intracardiac Echocardiography (ICE).Medical Surgical
CardiovascularElectrophysiology (EP) Catheter Solutions: Ablation Catheters, High-Density Mapping Catheters, and Intracardiac Echocardiography (ICE).Medical Surgical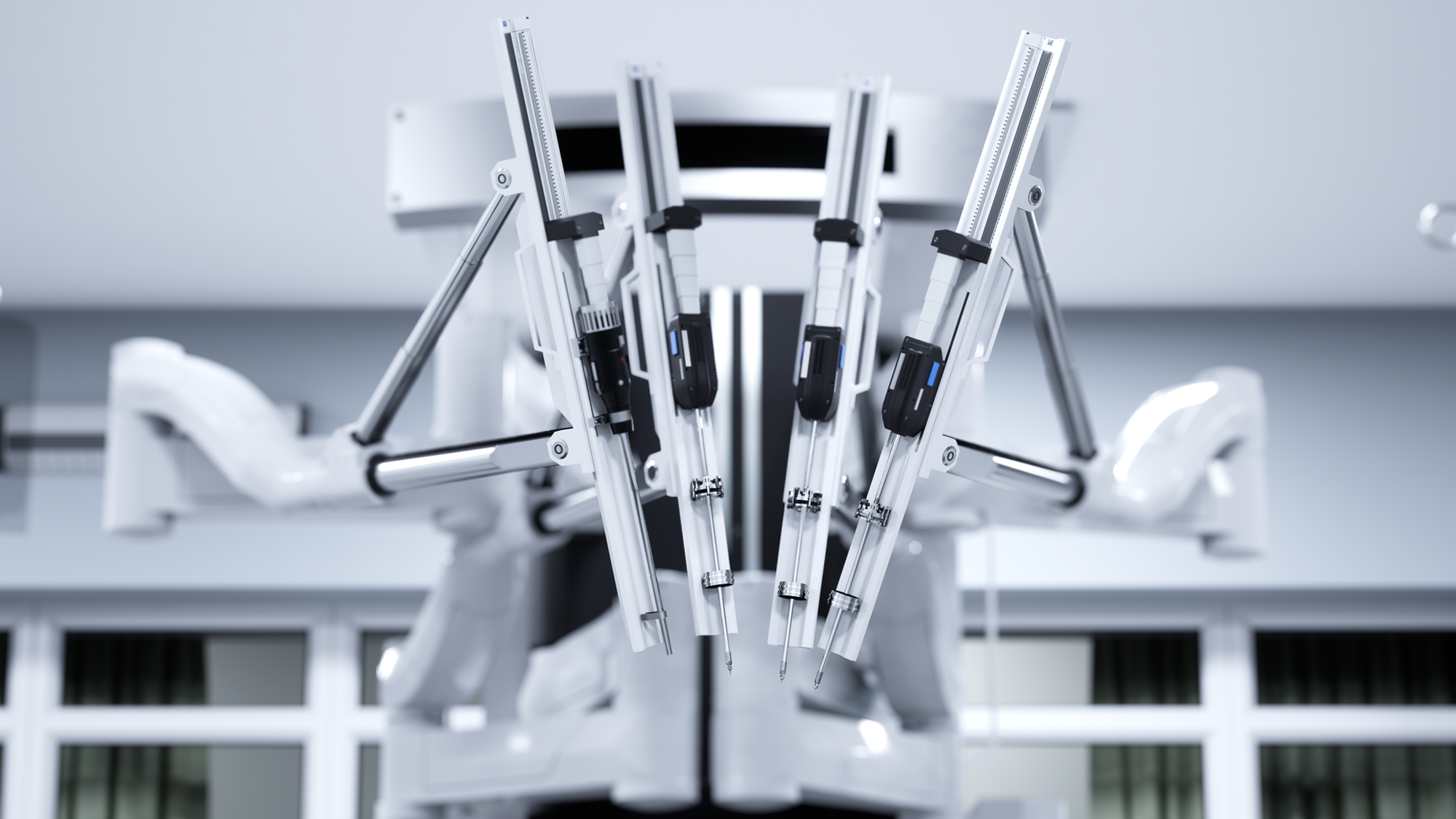 Surgical SolutionsProfessional Manufacturer of Minimally Invasive Surgical Products: Ultrasonic Scalpels, Staplers, Ligating Clips, Sterile-grade/Implant-grade Injection Components and More.Ophthalmology & ENT
Surgical SolutionsProfessional Manufacturer of Minimally Invasive Surgical Products: Ultrasonic Scalpels, Staplers, Ligating Clips, Sterile-grade/Implant-grade Injection Components and More.Ophthalmology & ENT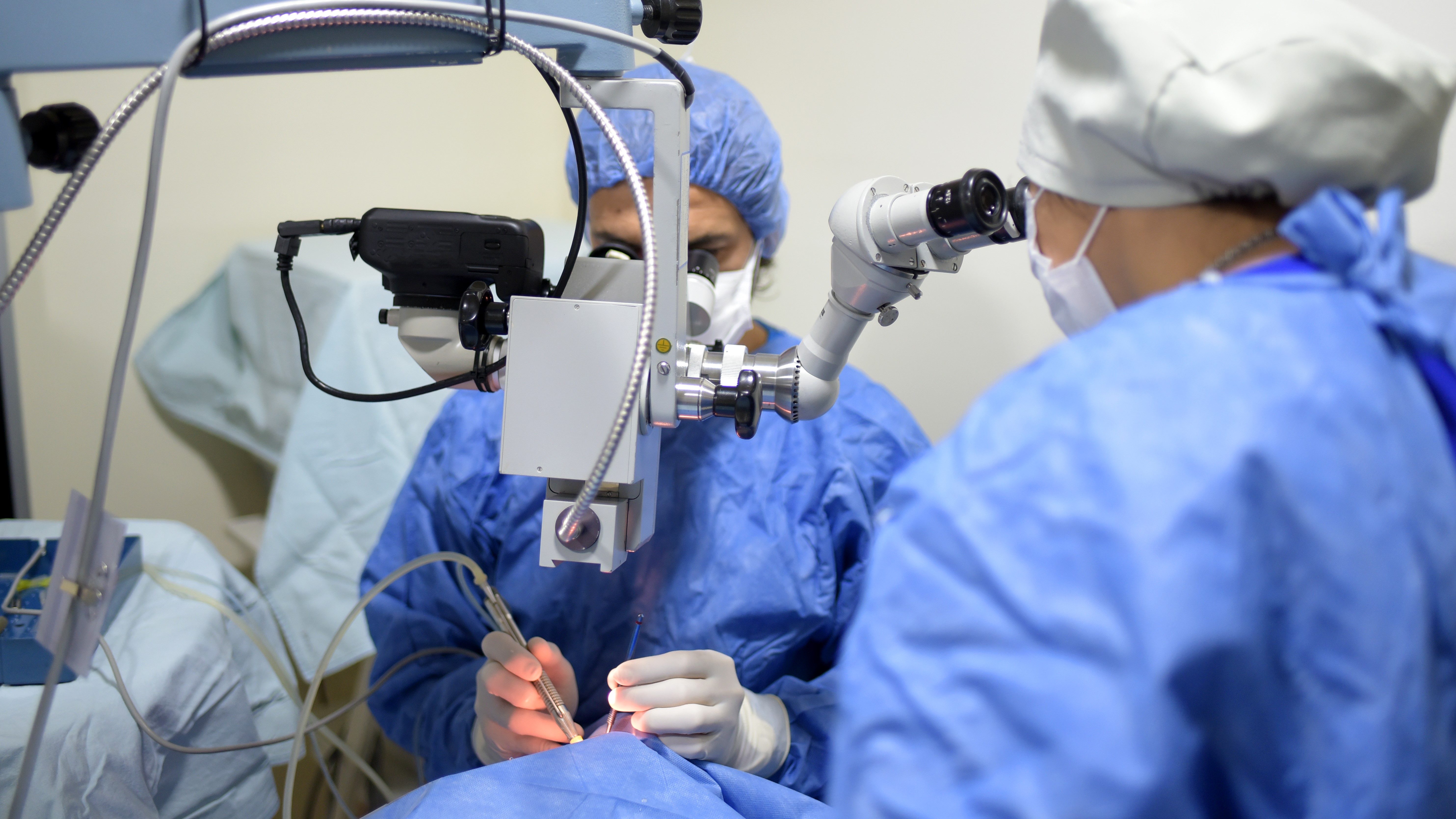 Ophthalmology & ENTOphthalmic High-Value Consumables: Phacoemulsification Disposables, IOL Delivery Systems and More.Respiratory
Ophthalmology & ENTOphthalmic High-Value Consumables: Phacoemulsification Disposables, IOL Delivery Systems and More.Respiratory Respiratory CareManufacturer of Respiratory Testing Equipment & Consumables: Ventilator Consumables, Pulmonary Function Test Systems, Disposable Filters, and More.Comprehensive Medical
Respiratory CareManufacturer of Respiratory Testing Equipment & Consumables: Ventilator Consumables, Pulmonary Function Test Systems, Disposable Filters, and More.Comprehensive Medical Comprehensive MedicalComprehensive manufacturing support for medical devices in multiple fields, from enteral nutrition and brain-computer interfaces to female reproductive health.Consumer Health
Comprehensive MedicalComprehensive manufacturing support for medical devices in multiple fields, from enteral nutrition and brain-computer interfaces to female reproductive health.Consumer Health Consumer HealthExtensive experience in the mass production of consumer products, covering areas such as baby and maternity products, consumer electronics, drinking water bottles, personal care, etc.
Consumer HealthExtensive experience in the mass production of consumer products, covering areas such as baby and maternity products, consumer electronics, drinking water bottles, personal care, etc. -
News and Events
Company News
 Company NewsStay updated with our latest milestones and industry impact.Marketing Activities
Company NewsStay updated with our latest milestones and industry impact.Marketing Activities Marketing ActivitiesWe actively engage with clients, partners, and industry peers through diversified market initiatives to shape the future together. Join us to explore collaboration opportunities.
Marketing ActivitiesWe actively engage with clients, partners, and industry peers through diversified market initiatives to shape the future together. Join us to explore collaboration opportunities. -
Careers
Life at MeHow
 Life at MeHowInnovating for Life and Health — Join Mehow Medical on a Journey Toward a Better Future.Jobs
Life at MeHowInnovating for Life and Health — Join Mehow Medical on a Journey Toward a Better Future.Jobs JobsExplore opportunities that match your ambition — apply now and begin a new chapter in your career with purpose and excellence. Join us in shaping the future of the global health industry.Students
JobsExplore opportunities that match your ambition — apply now and begin a new chapter in your career with purpose and excellence. Join us in shaping the future of the global health industry.Students StudentsYouth Knows No Limits — Begin Your Career Journey with Us! With learning and innovation as your wings, and dedication as your sail, let’s define your first destination together.
StudentsYouth Knows No Limits — Begin Your Career Journey with Us! With learning and innovation as your wings, and dedication as your sail, let’s define your first destination together.




